 Making the difference.
Making the difference.
e
T&T Scientific Corp.
SERVICES
- Formulation and Discovery
- Preclinical Development
- Clinical Trials Tech Transfer & GMP Manufacturing
- Commercial Manufacturing, Aseptic Fill and Finish
Get Your Proposal
TECHNOLOGIES
- NanoSizer FLOW Manufacturing
- NanoSizer Extrusion
- NanoSizer AUTO Technology
- NanoSizer Consumables
- Custom Nanoparticle Formulations
- ProFECT Formulation Technologies
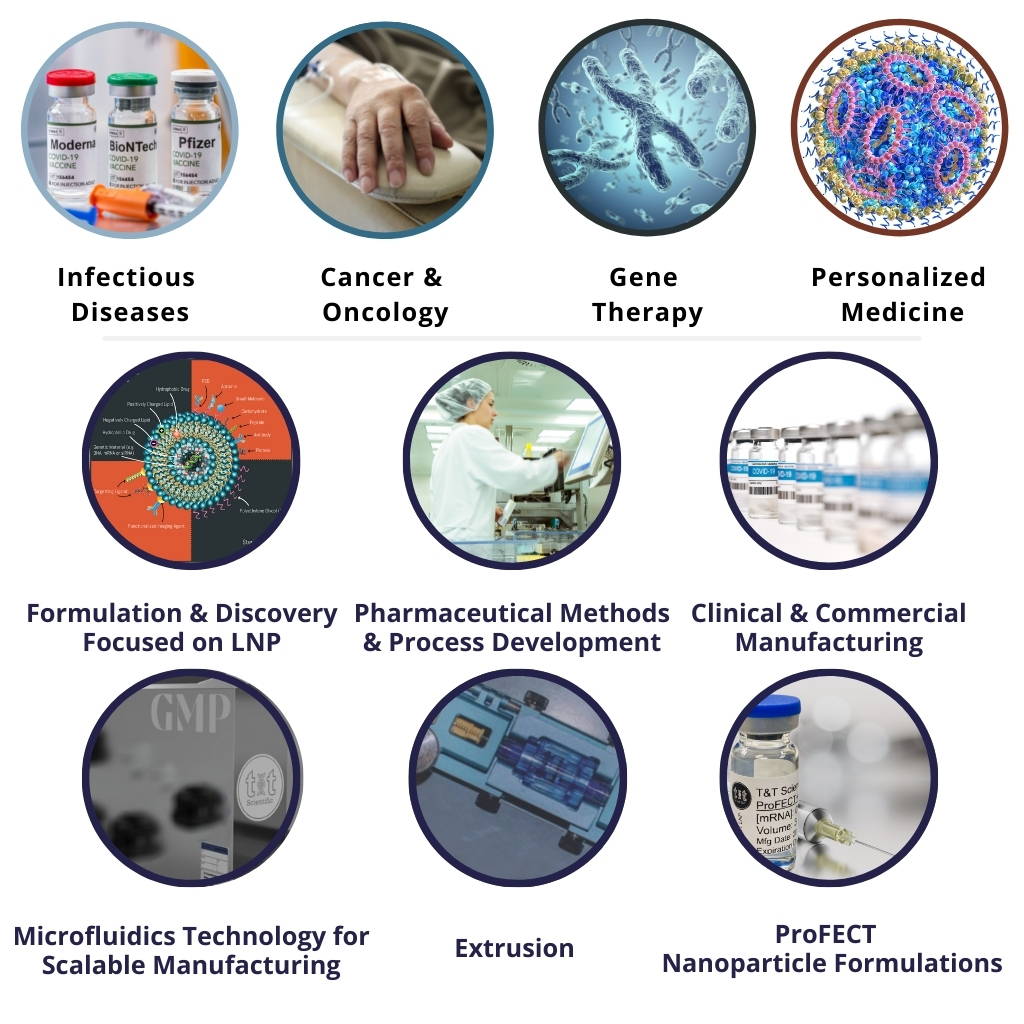
The Future of Translational Nanomedicines:
CRO & CDMO with Pharmaceutical Drug Delivery
Formulations and Manufacturing: Technologies and Services
Lipid-Based Pharmaceutical Drug Products including Lipid Nanoparticles (LNP's), Liposomes, and Lipoplexes
Hybrid Lipid-Polymer Drugs, and Polymer-Based Delivery of API's such as mRNA & Proteins
Formulation and Manufacturing of Vaccines and Therapeutics
Speciality and Expertise on Delivery System for APIs:
mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides,
siRNA, DNA, Small and Large Molecules
Manufacturing Technologies
CDMO Manufacturing Services
Discovery and CRO Services
Formulation Technologies
T&T Scientific
T&T Scientific is a technology and service company focused on formulation and manufacturing of pharmaceutical nanomedicines. T&T Scientific has three main core departments: 1) Contract Research, Development and cGMP manufacturing. 2) Manufacturing Platform All-Inclusive Technologies for In-house or Sales. and 3) Lipid Nanoparticle Formulation Discovery, Optimizations, and Technologies. T&T Scientific has quality system managed by quality team and electronic quality system (Qualio). T&T Scientific quality department insures the quality of both drug products, and technology transfers to and from T&T Scientific. Moreover, the company has established a practical education platform. This platform connects the world in the field of nanomedicine therapeutics and vaccines for the most effective dissemination of information in the field.
T&T Scientific is a technology and service company focused on formulation and manufacturing of pharmaceutical nanomedicines. T&T Scientific has three main core departments: 1) Contract Research, Development and cGMP manufacturing. 2) Manufacturing Platform All-Inclusive Technologies for In-house or Sales. and 3) Lipid Nanoparticle Formulation Discovery, Optimizations, and Technologies. T&T Scientific has quality system managed by quality team and electronic quality system (Qualio). T&T Scientific quality department insures the quality of both drug products, and technology transfers to and from T&T Scientific. Moreover, the company has established a practical education platform. This platform connects the world in the field of nanomedicine therapeutics and vaccines for the most effective dissemination of information in the field.
T&T Scientific Corporation’s Quality Management System takes it guidance from 21 CFR Part 820 Quality System Regulations and complies all FDA GMP regulatory requirements. These requirements include 21 CFR Part 210, 21 CFR Part 211, and 21 CFR Part 11. The company claims no exclusions from the 21 CFR Part 820, 201, 211, 11 at 7140 Regal Lane, Knoxville, TN 37918.
Quality Policies at T&T Scientific are: 1) Foster a quality mindset that promotes a passion for continual improvement. 2) Ensure compliance with all applicable regulatory requirements. 3) Guarantee safe and effective products and services that fulfill customers needs.
T&T Scientific Quality
T&T Scientific is a full cGMP facility under one roof. T&T Scientific was founded to provide end users and customers with liposomal and lipid-nanoparticle equipment, research and development, and manufacturing services. And ultimately to help them achieve their goals in bringing life changing and life saving technologies to the world. Each department is a discrete functional unit of the organization, and a quality centric approach is at the core of all that we do. T&T’s Quality Operations are governed by the site’s Quality Manual. T&T Scientific Corporation’s Quality Management System takes it guidance from 21 CFR Part 820 Quality System Regulations and complies all FDA GMP regulatory requirements. These requirements include 21 CFR Part 210, 21 CFR Part 211, and 21 CFR Part 11. The company claims no exclusions from the 21 CFR Part 820, 201, 211, 11 at 7140 Regal Lane, Knoxville, TN 37918.
Services
T&T Scientific is a full cGMP operation under one umbrella. T&T operations unit covers projects from early discovery and optimization of drug products, delivery systems to pharmaceutical manufacturing process and analytical methods development, to clinical and commercial manufacturing. T&T is registered with the FDA and producing clinical materials for multiple pharmaceutical and bio technology companies. T&T Scientific comprehensive services are categorized to following:
Formulation and delivery system discovery and optimization
Analytical Methods, and Manufacturing Processes Development
Preclinical Pharmaceutical Development and Engineering Batch
Phase I, II Clinical Trial Manufacturing
Phase III Clinical Trial Batch Manufacturing
Commercial Batch Manufacturing
Sterile Filtration, Fill and Finish with Labeling for All of Above
Formulation and delivery system discovery and optimization Analytical Methods, and Manufacturing Processes Development Preclinical Pharmaceutical Development and Engineering Batch Phase I, II Clinical Trial Manufacturing Phase III Clinical Trial Batch Manufacturing Commercial Batch Manufacturing Sterile Filtration, Fill and Finish with Labeling for All of Above
T&T Scientific is constantly innovating in the space of nanomedicine manufacturing and formulations. T&T has expanded its manufacturing technology to highly scalable, precise, low cost, and small footprint solvent injection technologies, NanoSizer Flow, starting with patent-approved continuous extrusion technology. The solvent injection technology is being widely used in COVID vaccines and other gene therapy manufacturing applications. Moreover, the formulation department at T&T develops novel Lipid Nanoparticle (LNP) formulations for a wide variety of active pharmaceutical ingredients (APIs), including mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides, siRNA, DNA, Small and Large Molecules. ProFECT is the brand for T&T's lipid, polymer, and hybrid nanoparticle formulations.
Technologies
T&T Scientific is constantly innovating in the space of nanomedicine manufacturing and formulations. T&T has expanded its manufacturing technology to highly scalable, precise, low cost, and small footprint solvent injection technologies, NanoSizer Flow, starting with patent-approved continuous extrusion technology. The solvent injection technology is being widely used in COVID vaccines and other gene therapy manufacturing applications. Moreover, the formulation department at T&T develops novel Lipid Nanoparticle (LNP) formulations for a wide variety of active pharmaceutical ingredients (APIs), including mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides, siRNA, DNA, Small and Large Molecules. ProFECT is the brand for T&T's lipid, polymer, and hybrid nanoparticle formulations.

Terms and Conditions
Right of Withdrawal
Privacy Policy
Disclaimer Statement
Search
Terms of Service
Refund policy
Snapchat
Google Plus
Tumblr
YouTube
Vimeo
Fancy
© 2022, T&T Scientific Corp.
Payment methods
en English
Unique experiences to drive engagement
T&T Scientific has introduced a low-cost, single-use liposome extrusion device that simplifies the process of preparing liposomes for research laboratories, manufacturing facilities, and clinical settings around the globe.
Unlike anything available to date, NanoSizerTM extruders ensure cleanliness and sterility while reducing the overall time of use from 45-60 minutes to 2 minutes. The low dead-volume design saves research dollars by reducing the amount of valuable biological material lost with conventional syringe filters.
Liposomes, polymerosomes, or related therapeutics can now be safely and effectively prepared in college science labs, academic research labs, pharmaceutical labs and production facilities labs, and even in hospitals and clinical settings.
NanoSizerTM extrusion technology from T&T brings confidence, convenience, and simplicity to doctors and scientists all around the world.
Products by T&T Scientific
50 nm NanoSizer MINI Liposome Extruder
100 nm NanoSizer MINI Liposome Extruder
50 Extrusion Syringes and 50 Low Dead-Volume Needles
Liposome Extrusion Holder/Heat Block
Complete NanoSizer Extrusion Kit (all pore sizes)

Useful options
Beautiful snippets
Amazing pages
Outstanding images
Grow with Us
- The Future of Translational Nanomedicines:
- CRO & CDMO with Pharmaceutical Drug Delivery
- Formulations and Manufacturing: Technologies and Services
- Lipid-Based Pharmaceutical Drug Products including Lipid Nanoparticles (LNP's), Liposomes, and Lipoplexes
- Hybrid Lipid-Polymer Drugs, and Polymer-Based Delivery of API's such as mRNA & Proteins
- Formulation and Manufacturing of Vaccines and Therapeutics
Speciality and Expertise on Delivery System for APIs:
- mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides,
- siRNA, DNA, Small and Large Molecules
- Manufacturing Technologies
- CDMO Manufacturing Services
- Discovery and CRO Services
- Formulation Technologies
T&T Scientific
T&T Scientific is a technology and service company focused on formulation and manufacturing of pharmaceutical nanomedicines. T&T Scientific has three main core departments: 1) Contract Research, Development and cGMP manufacturing. 2) Manufacturing Platform All-Inclusive Technologies for In-house or Sales. and 3) Lipid Nanoparticle Formulation Discovery, Optimizations, and Technologies. T&T Scientific has quality system managed by quality team and electronic quality system (Qualio). T&T Scientific quality department insures the quality of both drug products, and technology transfers to and from T&T Scientific. Moreover, the company has established a practical education platform. This platform connects the world in the field of nanomedicine therapeutics and vaccines for the most effective dissemination of information in the field.
T&T Scientific is a technology and service company focused on formulation and manufacturing of pharmaceutical nanomedicines. T&T Scientific has three main core departments: 1) Contract Research, Development and cGMP manufacturing. 2) Manufacturing Platform All-Inclusive Technologies for In-house or Sales. and 3) Lipid Nanoparticle Formulation Discovery, Optimizations, and Technologies. T&T Scientific has quality system managed by quality team and electronic quality system (Qualio). T&T Scientific quality department insures the quality of both drug products, and technology transfers to and from T&T Scientific. Moreover, the company has established a practical education platform. This platform connects the world in the field of nanomedicine therapeutics and vaccines for the most effective dissemination of information in the field.
T&T Scientific Corporation’s Quality Management System takes it guidance from 21 CFR Part 820 Quality System Regulations and complies all FDA GMP regulatory requirements. These requirements include 21 CFR Part 210, 21 CFR Part 211, and 21 CFR Part 11. The company claims no exclusions from the 21 CFR Part 820, 201, 211, 11 at 7140 Regal Lane, Knoxville, TN 37918.
Quality Policies at T&T Scientific are: 1) Foster a quality mindset that promotes a passion for continual improvement. 2) Ensure compliance with all applicable regulatory requirements. 3) Guarantee safe and effective products and services that fulfill customers needs.
T&T Scientific Quality
T&T Scientific is a full cGMP facility under one roof. T&T Scientific was founded to provide end users and customers with liposomal and lipid-nanoparticle equipment, research and development, and manufacturing services. And ultimately to help them achieve their goals in bringing life changing and life saving technologies to the world. Each department is a discrete functional unit of the organization, and a quality centric approach is at the core of all that we do. T&T’s Quality Operations are governed by the site’s Quality Manual. T&T Scientific Corporation’s Quality Management System takes it guidance from 21 CFR Part 820 Quality System Regulations and complies all FDA GMP regulatory requirements. These requirements include 21 CFR Part 210, 21 CFR Part 211, and 21 CFR Part 11. The company claims no exclusions from the 21 CFR Part 820, 201, 211, 11 at 7140 Regal Lane, Knoxville, TN 37918.
Services
T&T Scientific is a full cGMP operation under one umbrella. T&T operations unit covers projects from early discovery and optimization of drug products, delivery systems to pharmaceutical manufacturing process and analytical methods development, to clinical and commercial manufacturing. T&T is registered with the FDA and producing clinical materials for multiple pharmaceutical and bio technology companies. T&T Scientific comprehensive services are categorized to following:
Formulation and delivery system discovery and optimization
Analytical Methods, and Manufacturing Processes Development
Preclinical Pharmaceutical Development and Engineering Batch
Phase I, II Clinical Trial Manufacturing
Phase III Clinical Trial Batch Manufacturing
Commercial Batch Manufacturing
Sterile Filtration, Fill and Finish with Labeling for All of Above
Formulation and delivery system discovery and optimization Analytical Methods, and Manufacturing Processes Development Preclinical Pharmaceutical Development and Engineering Batch Phase I, II Clinical Trial Manufacturing Phase III Clinical Trial Batch Manufacturing Commercial Batch Manufacturing Sterile Filtration, Fill and Finish with Labeling for All of Above
T&T Scientific is constantly innovating in the space of nanomedicine manufacturing and formulations. T&T has expanded its manufacturing technology to highly scalable, precise, low cost, and small footprint solvent injection technologies, NanoSizer Flow, starting with patent-approved continuous extrusion technology. The solvent injection technology is being widely used in COVID vaccines and other gene therapy manufacturing applications. Moreover, the formulation department at T&T develops novel Lipid Nanoparticle (LNP) formulations for a wide variety of active pharmaceutical ingredients (APIs), including mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides, siRNA, DNA, Small and Large Molecules. ProFECT is the brand for T&T's lipid, polymer, and hybrid nanoparticle formulations.
Technologies
T&T Scientific is constantly innovating in the space of nanomedicine manufacturing and formulations. T&T has expanded its manufacturing technology to highly scalable, precise, low cost, and small footprint solvent injection technologies, NanoSizer Flow, starting with patent-approved continuous extrusion technology. The solvent injection technology is being widely used in COVID vaccines and other gene therapy manufacturing applications. Moreover, the formulation department at T&T develops novel Lipid Nanoparticle (LNP) formulations for a wide variety of active pharmaceutical ingredients (APIs), including mRNA, Self Amplifying RNA, Proteins, Antibodies, Peptides, siRNA, DNA, Small and Large Molecules. ProFECT is the brand for T&T's lipid, polymer, and hybrid nanoparticle formulations.

Beginner
$ 35 .00
/ month- Basic sales & marketing for up to 2 users
- Account & Sales management
- No customization
- No support
Professional
$ 65 .00
/ month- Complete CRM for any size team
- Access to all modules
- Limited customization
- Email support
Expert
$ 125 .00
/ month- Unlimited CRM power and support
- Access to all modules and features
- Unlimited customization
- 24/7 support